31+ Bcl3 Molecular Geometry Background
31+ Bcl3 Molecular Geometry
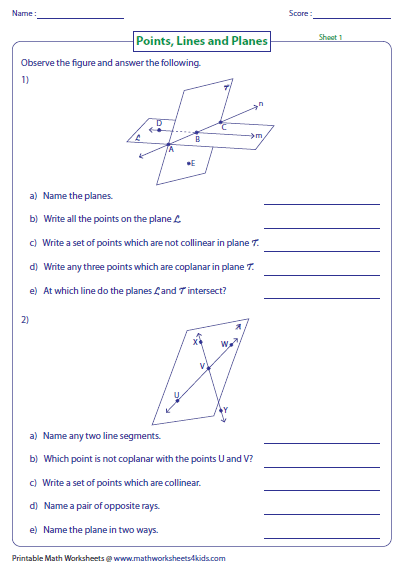
Background. What are the rules of valence shell electron pair repulsion (vsepr)? It has trigonal planar geometry.
This video tutorial will explain how to draw the lewis dot structure and molecular geometry for boron trichloride (bcl3).
This is free chemistry help for you. Number of electron pairs around central atom. 1) determine the electron geometry (eg) and molecular geometry(mg) of bcl3. 18) for which of the molecules is the molecular geometry (shape) the same as the vsepr electron domain arrangement (electron.



Posting Komentar untuk "31+ Bcl3 Molecular Geometry Background"