New Xef4 Molecular Geometry Full
New Xef4 Molecular Geometry
Full. The electron geometry is octahedral, while the molecular geometry is square planar, xenon has 6 bonding electron pairs, therefore the electron geometry of octahedral, but two of the pairs of electrons on the central atom are unbonded. Next draw the 3d molecular geometry using vsepr rules:
Data that may be obtained from a molecule's geometry includes the relative position of.
The electron geometry is octahedral, while the molecular geometry is square planar, xenon has 6 bonding electron pairs, therefore the central atom xenon in xef4 is sp3d2 hybridised suggesting that the gross geometry should be octahedral. 2 vsepr model • valence shell electron pair repulsion • a fancy name for a simple model. Molecules with the same chemical formula may have atoms arranged differently. Predict the electron domain geometry, molecular geometry, and bond angles of for each central atom in the ch3cooh structure.


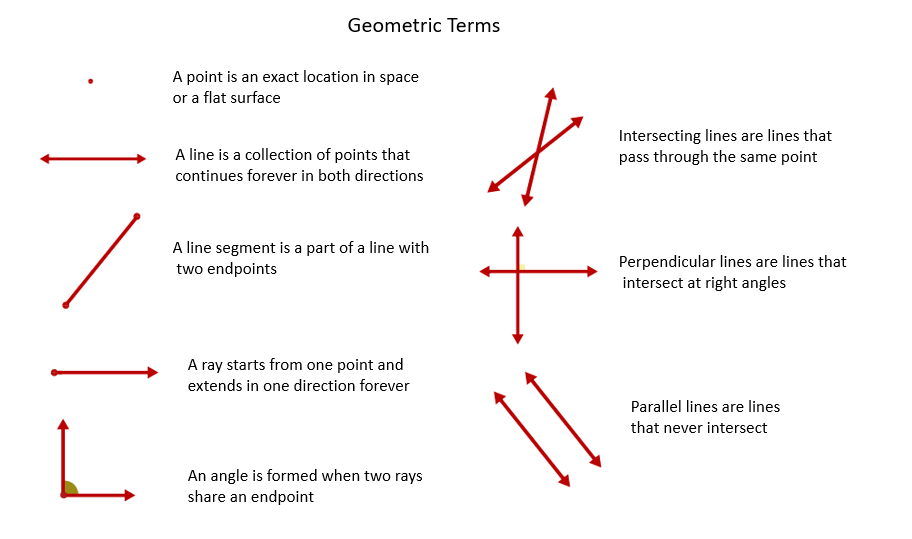

Posting Komentar untuk "New Xef4 Molecular Geometry Full"